

Advanced INNOVATIVE
PARTNERS
Making a Difference In
Patient’s Lives in The Renaissance of Radiopharmaceuticals

Our FOCUS
Theranostics
Drug Development
Preclinical Trials
Radiopharmaceuticals
Gallium-68
Diagnostics
Precision Medicine
Clinical Trials
Actinium-225
Fluorine- 18
Targeted Therapeutics
Licensing
INDs Enabling
Copper- 64
Lutetium-177

Welcome
Advanced Innovative Partners is a radiopharmaceutical company committed to advancing the field of healthcare through novel diagnostic and therapeutic innovations. We specialize in the development of technologies for oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures. Our mission is to provide clinicians with the tools they need to better understand and manage complex diseases, ultimately improving therapeutic outcomes.
Roseanne Satz, Chief Executive Officer

ABOUT US
Advanced Innovative Partners is a global clinical-stage biotechnology company developing diagnostic and therapeutic (theranostic) products utilizing targeted radiation. AIP has a robust pipeline for breast, lung, brain, and solid tumor cancers and rare diseases, supported by a reliable, secure global supply chain and collaborator network.
With expertise in biotechnology, nuclear medicine, and molecular biology, our team of experienced researchers, clinicians, and engineers are dedicated to developing next-generation diagnostic and therapeutic radiopharmaceuticals that meet the needs of patients and healthcare providers.

Mission
Our mission is to develop innovative diagnostic and therapeutic products that significantly improve patient outcomes and quality of life. We are committed to researching, developing and providing specialized radiopharmaceuticals, precision medicines, and biomedical solutions for hard-to-treat diseases.
VISION
Our vision is to transform patient care by being at the forefront of precision medicine and targeted therapies. We strive to develop breakthrough solutions that enable early detection and effective treatment of challenging diseases to extend and enhance life.


CORPORATE OVERVIEW
Advanced Innovative Partners (AIP) is a clinical-stage biotechnology company founded in 2017 with a highly skilled team that has developed patented platform technologies for treatment and clinical management in oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.
We are building a vertically integrated radiopharmaceutical company with our lead programs in Phase I/II/III clinical trials. We have established a leadership position in the emerging radiopharmaceutical diagnostics and therapeutics market space through a product-centric approach and have created a pipeline of multiple drugs in areas with significant market opportunities.


Revolutionizing MEDICINE
PET Scans - Nuclear Medicine
Oncologists and epidemiologists have utilized radiopharmaceuticals consisting of radioconjugated tracers in their medical practices for decades. PET (Positron Emission Tomography) scans employ these radiotracers as powerful diagnostic tools to identify cancers, infections, and other conditions by visually highlighting abnormal functional processes in the body. Beyond diagnosis, radiopharmaceuticals also show promise for targeted therapeutic applications.


OUR TECHNOLOGY
Our technology has a promising role in cancer and infectious diseases. AIP’s developments focus on understanding the role of integrins, a family of cell surface receptors, in both cancer progression and infectious disease pathogenesis. Using advanced imaging techniques, we track integrin signaling in tumor models to shed light on how these receptors influence key events in metastasis, including extravasation and colonization. This knowledge aids in the development of novel integrin-targeted therapies. In parallel, we explore integrin-pathogen interactions and how they can be leveraged to combat difficult-to-treat infections.
AIP has both developed and licensed an innovative portfolio of best-in-class products, formulation, and manufacturing processes. Our world-leading research and development teams are working to improve targeted radiation therapy (TRT), revolutionizing cancer treatment. AIP's approach in oncology involves the use of a peptide mimetic radio-conjugate utilizing specific isotopes for theragnostic: Gallium-68 as a diagnostic tool, and Lutetium-177 and Actinium-225 as therapeutic agents.
Certain radioisotopes possess physical characteristics that can be harnessed for diagnostic and staging purposes in the imaging of cancer or rare diseases. Meanwhile, radioisotopes emitting higher doses of alpha and beta radiation hold the potential for therapeutic applications in eradicating cancerous or diseased cells.


OUR SCIENCE
Our research utilizes advanced medical imaging technologies to improve the clinical management of rare pediatric diseases. Specifically, we are developing proprietary imaging agents that target key receptors, involved in cancer and rare pediatric orphan CNS diseases. Visualizing key receptors in patients enables earlier detection and better characterization of complex disease conditions. A current focus is on creating novel radio imaging agents to safely and effectively diagnose rare pediatric diseases that are difficult to monitor over progression and treatment.
Certain radioisotopes possess physical characteristics that can be harnessed for diagnostic and staging purposes in the imaging of cancer or rare diseases. Meanwhile, radioisotopes emitting higher doses of alpha and beta radiation hold the potential for therapeutic applications in eradicating cancerous or diseased cells.

HISTORY

Changing the way we treat diseases
Licensed NIH Technology,
Developed Chemistry Methodology
2018
Conducted Preclinical Studies, filed Orphan Applications, file Intellectual Property,
Performed Clinical Trials
2019
Conducted Clinical
Trials for Oncology Assets Submitted
Orphan Drug Applications
2020
Continued Clinical Trials, Production of
Platform Technology, Filed INDs,
Submitted Patents
2021
Orphan Applications filed, Patent
Awarded, Sepsis pre-clinical study,
Progressing to Cancer Trials
2022
Completed Toxicology for Platform Technology,
Production of Biomedical Countermeasure Drug,
IND Filings for Oncology and Rare Pediatric Diseases
2023
Management Team
Chairmand and CSO
Colm King
Chief Financial Officer
VP of Clinical Research and Development
VP of Strategic Development
Director of Regulatory of Affairs
Hamid Zolata, M.D. Ph.D.
Chief Medical Officer

Advisors
Ann Harrington
Honorable
Thom Tulip, Ph.D.
Gerardo Morales
Nirmal Ganguly, M.D. Ph.D.
Paul Crowe
Michael Burns, DDS
Sandy McEwan, M.D.

OUR TARGETS
TARGETS
AIP is developing a diverse portfolio of technologies aimed at addressing unmet medical needs including oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures. Our focus is on developing innovative therapeutic and diagnostic solutions that are closely integrated and personalized to patients.
Oncology
Rare Pediatric Diseases
Infectious Disease
Biomedical Countermeasures

ONCOLOGY

Our robust oncology portfolio comprises multiple theragnostic agents to enable precise diagnosis and treatment across various cancer types. These technologies are engineered to enhance patient outcomes through early and accurate cancer detection, as well as personalized therapeutic plans tailored to each patient's unique needs. By transforming detection and treatment, we aim to significantly improve survival rates and quality of life for cancer patients

RARE DISEASES

Our rare disease portfolio features diagnostic agents intended to address a variety of rare and disabling childhood conditions. These novel radiopharmaceuticals are engineered to facilitate earlier and more precise diagnosis, as well as tailored treatment plans that can dramatically improve quality of life for affected children and their families. By targeting critical unmet needs, we aim to transform the standard-of-care for numerous rare pediatric diseases.
INFECTIOUS DISEASES
Our infectious disease pipeline features diagnostic and therapeutic radiopharmaceuticals to facilitate rapid, precise diagnosis of various pathogenic infections, as well as personalized treatments tailored to each patient. These technologies are especially critical for emerging infectious diseases and bioterrorism threats. By transforming detection and treatment, we aim to curb outbreaks and save lives through accurate, prompt diagnosis and tailored patient care.

BIOMEDICAL COUNTERMEASEURES

Our biomedical countermeasures pipeline comprises specialized drugs to bolster medical countermeasures against biological, radiological and nuclear dangers. These technologies are engineered to effectively treat acute radiation syndrome and safeguard armed forces and civilians from radiological exposure and contamination. By transforming preparedness and response, we aim to mitigate fallout and mortality from weapons of mass destruction.

MARkET OPPORTUNITY
There is a significant unmet need and market opportunity to develop innovative therapies across oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.Cancer remains a leading cause of death globally, with the economic burden expected to reach $458 billion by 2030 in the United States alone. Rare pediatric diseases also present unique R&D challenges and impact millions of young lives. Meanwhile, the constant threat of drug resistant infectious diseases emphasizes the need for improved preparedness through novel diagnostics and therapeutics.
ONCOLOGY
$5B
Rare DISEASEs
$1B
INFECTIOUS DISEASEs
$6B
BIOMEDICAL
COUNTER
MEASURES

OUR PIPELINE
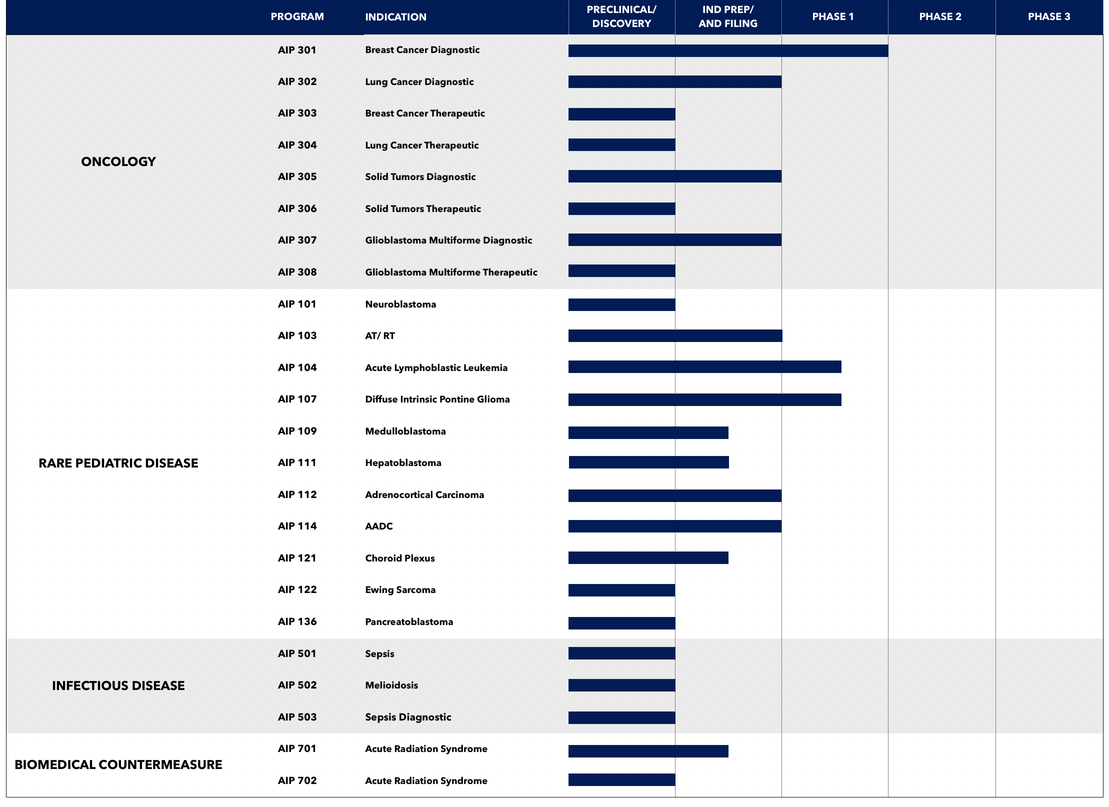

We have built a portfolio of potential drug and development candidates spanning various stages of progress.
This includes mid-stage clinical programs, development programs past IND-enabling studies, and early discovery programs.
We file Investigational New Drug (IND) applications and advance our most promising programs into formal clinical development.
A diverse portfolio of proven products

CLINICAL TRIALS
Clinical trials are research investigations designed to determine if new treatments are safe, effective, and superior to existing options. These studies adhere to strict oversight and careful evaluation protocols.

Shiva Study
NCT04480619
Heroine Study
NCT 04469127
DIPLO Study
Atari Study
Alva Study
AIP is dedicated to expediting the discovery, development, and worldwide availability of transformative therapies. We concentrate on performing the clinical trials mandated by regulatory bodies to ascertain the potential risks and benefits of our investigational products. By obtaining regulatory approval, we aim to make our medications accessible to patients.
CORE ETHICS
Innovation
Development
Regulatory Affairs
Innovation is the key to AIP developing novel imaging agents and targeted therapies, that can revolutionize patient treatment. By incorporating research, creativity, and a willingness to experiment, our team has made incremental improvements by radical breakthroughs in identifying patients who would benefit from our treatments.
Cooperation
Cooperation is essential to building AIP's partnerships and collaborations with other companies, academic institutions and healthcare providers. As a result, researchers can gain access to new technologies and expertise, share resources, to accelerate the development of effective novel therapies.
Development is a critical component of advancing the field of AIP's theranostics which involves design, and analysis of clinical and preclinical studies. Our team is implementing new ideas, methods, products, and therapies that can be used to identify patients who would benefit from these treatments.
Intellectual Property
Intellectual property (IP) protection is critical for companies developing diagnostics and therapeutics. By protecting our inventions, and discoveries through patents, trademarks, and other forms of IP protection, AIP establishes a competitive advantage in the market to drive progress and innovation.
Regulatory affairs play a critical role in obtaining approvals for AIP's products and technologies, to ensure that our products meet safety and efficacy standards. AIP works closely with regulatory agencies and adhere to strict cGMP regulatory controls imposed by governmental authorities managing the complex process.
Commercialization
Commercialization of our product portfolio involves partnering with pharmaceutical companies to develop effective sales and marketing campaigns generating revenue through licensing of our intellectual property (IP) , which will enable AIP to monetize products in development.

OUR PURPOSE
We exist to pioneer targeted therapies and advance disease detection and treatment through an integrated approach spanning proprietary tech platforms, strategic partnerships, and a patient-centric focus. Our purpose is to profoundly help patient journeys by enabling accurate diagnoses sooner and providing more powerful yet gentler treatments, giving patients the best chance for better outcomes. We want to make a meaningful difference for those impacted by complex, aggressive, rare and infectious diseases around the world.


Contact Information
info@advancedinnovativepartners.com
www.advancedinnovativepartners.com
Telephone: 561 757-8666
https://www.linkedin.com/company/aip-advanced-innovative-partners/
